Schedule Your Dermal Filler Appointment with Dr. Laura Geige at It’s Me and You Clinic
Understanding the Risks and Benefits
Tear trough fillers are a popular cosmetic treatment used to reduce the appearance of dark circles and hollows under the eyes. While they can be an effective solution for many individuals, it’s essential to understand the potential risks and benefits before making a decision.
The benefits of tear trough fillers include:
- Quick and relatively painless procedure
- Minimally invasive, with no downtime required
- Precise control over the amount of filler used
- Temporary solution that can be easily reversed if needed
However, there are also potential risks and complications associated with tear trough fillers:
- Bleeding or bruising at the injection site
- Swelling, redness, or itching at the treated area
- Infection or abscess formation
- Asymmetrical or uneven results
- Temporary numbness or sensitivity in the eyelid or surrounding area
- Rarely, more severe complications such as blood clots, stroke, or vision loss have been reported
Common complications that may arise from tear trough fillers include:
- Temporary bruising: This is the most common complication and usually resolves on its own within a few days.
- Swelling and redness: These symptoms can be caused by the body’s reaction to the filler material or from the needle itself. They typically resolve within a week or two.
- Asymmetrical results: This can occur if the filler is not used in precise quantities or if there is an uneven distribution of the product.
- Infection: While rare, it’s essential to monitor the treated area for signs of infection, such as increased redness, swelling, or discharge.
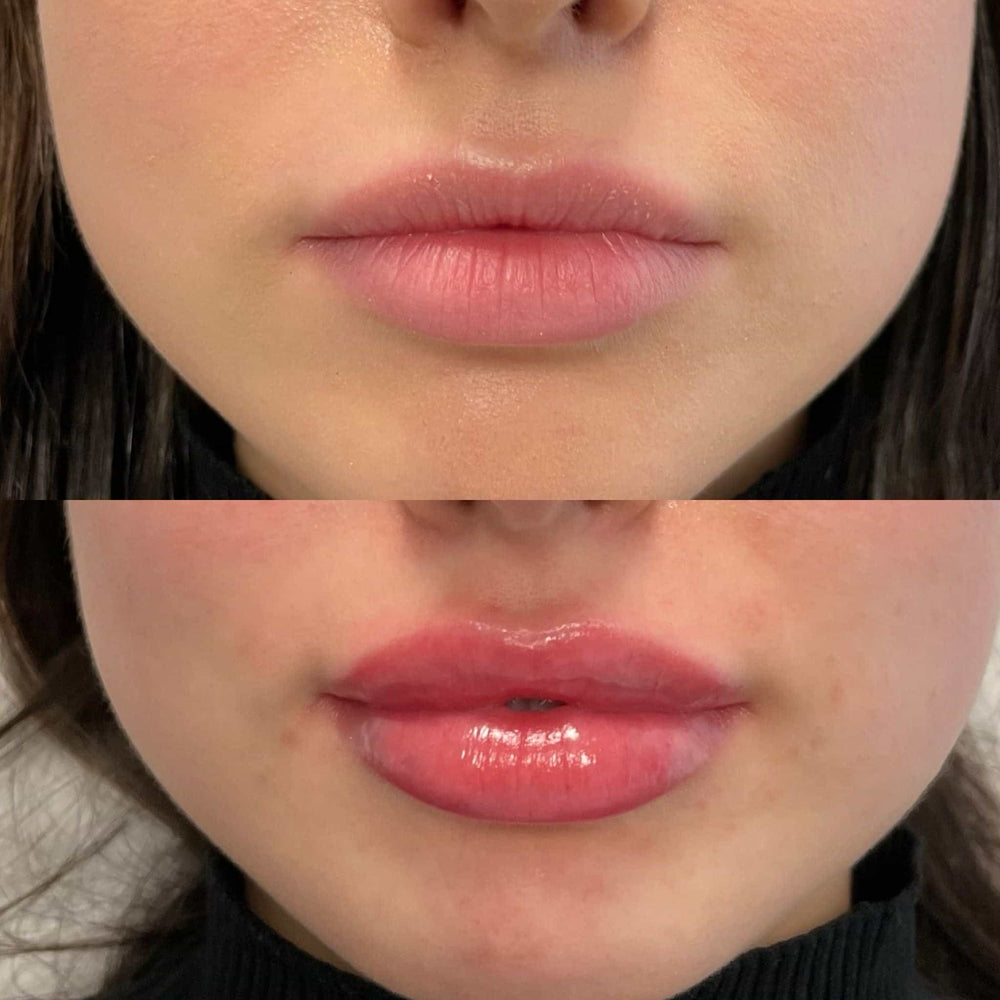
Long-term risks and benefits of tear trough fillers should also be considered:
- Temporary effects may last from a few months to several years
- The need for repeated treatments to maintain the desired effect
- Potential long-term complications, such as foreign body reactions or granulomas
It’s crucial to note that individual results may vary, and the risks associated with tear trough fillers can be minimized by choosing a qualified and experienced healthcare professional for treatment.
The safety and efficacy of tear trough fillers have become increasingly popular among individuals seeking to address nasolabial folds, hollows under the eyes, and other facial concerns.
However, like any cosmetic treatment, tear trough fillers carry both risks and benefits that must be carefully considered before making a decision.
Risks associated with tear trough fillers include, but are not limited to, allergic reactions, skin irritation, swelling, redness, bruising, and temporary numbness or tingling in the treated area.
More serious complications, although rare, can occur, including eyelid drooping, vision problems, eye pain, and infection.
The risk of adverse reactions may be exacerbated by certain pre-existing conditions, such as autoimmune disorders, eczema, or previous facial fillers.
Additionally, individuals taking certain medications, including blood thinners, should exercise caution when considering tear trough fillers to minimize the risk of bleeding complications.
On the other hand, the benefits of tear trough fillers are numerous and well-documented.
These fillers can effectively reduce the appearance of nasolabial folds, hollows under the eyes, and other facial creases, resulting in a more youthful and rejuvenated appearance.
Tear trough fillers can also enhance facial symmetry, improve facial contour, and boost self-confidence.
Furthermore, tear trough fillers are relatively quick and easy to administer, with most procedures taking no longer than 30 minutes to an hour.
Results from tear trough fillers typically last between 6-12 months, allowing for a convenient and flexible treatment schedule.
Moreover, the temporary nature of these fillers means that any unwanted side effects or complications can be easily addressed with minimal downtime.
Informed decision-making is key to maximizing the benefits of tear trough fillers while minimizing potential risks.
It is essential to choose a qualified and experienced practitioner who uses high-quality products and follows proper technique to ensure safe and effective results.
Before undergoing treatment, it is crucial to thoroughly discuss your medical history, concerns, and expectations with a trained professional to determine if tear trough fillers are the right choice for you.
A thorough consultation and follow-up care can help mitigate potential risks and maximize the benefits of this popular cosmetic treatment.
“Understanding the Risks and Benefits” of Tear Trough Fillers
Tear trough fillers, such as hyaluronic acid (HA) or calcium hydroxylapatite (CaHA), are a popular cosmetic treatment used to address concerns of dark circles, hollow eyes, and sagging eyelids. While generally considered safe, like any medical procedure, tear trough fillers carry potential risks and benefits that need to be carefully weighed.
Risks:
-
Bruising and swelling at the injection site, which can last for several days:
This is a common side effect of tear trough filler injections, particularly with the use of HA fillers. The bruising and swelling can range from mild to severe and may be more noticeable in people who are prone to bruising or have a history of bleeding disorders.
-
Infection at the injection site:
As with any invasive medical procedure, there is a small risk of infection with tear trough fillers. This can lead to redness, swelling, and increased pain at the injection site.
-
Nerve damage or numbness:
There is a rare but possible risk of nerve damage or numbness in the forehead or eyelid area due to the injection of fillers. In most cases, this symptom resolves on its own within a few weeks.
-
Allergic reactions:
Although rare, some people may be allergic to certain components of tear trough fillers, such as the preservatives used in HA products. Symptoms of an allergic reaction can range from mild hives to life-threatening anaphylaxis.
Benefits:
-
Improved appearance:
Tear trough fillers can effectively reduce the appearance of dark circles, hollow eyes, and sagging eyelids, resulting in a more youthful and radiant look.
-
Non-surgical option:
Unlike surgical blepharoplasty or other invasive procedures, tear trough fillers offer a minimally invasive alternative for addressing these concerns.
-
Quick recovery:
Most people can return to their normal activities immediately after the procedure, with minimal downtime required.
-
Customizable results:
The effects of tear trough fillers can be customized to achieve a more natural-looking result by adjusting the amount of filler used and the number of injections needed.
-
Pain management:
Modern pain management techniques, such as local anesthesia or numbing creams, minimize discomfort during and after the procedure.
To minimize the risks associated with tear trough fillers, it is essential to choose a qualified and experienced healthcare professional to administer the treatment. Additionally, following post-procedure instructions carefully can help ensure optimal results and reduce the likelihood of complications.
In order to thoroughly assess the safety of *tear trough filler*, it’s essential to delve into its potential risks and benefits, as well as a possible side effect – pain or numbness at the injection site.
The main goal of tear trough fillers is to temporarily address concerns about hollow under-eye appearance by injecting materials like hyaluronic acid, calcium hydroxylapatite, or poly-L-lactic acid into the orbital bone beneath the **cheekbone**. This procedure can enhance the overall aesthetic appeal of the face.
When considering the risks associated with tear trough fillers, patients should be aware that there are potential complications, including:
- Pain or numbness at the injection site (paresthesia), which typically subsides within a few hours;
- Infection;
- Scarring;
- Bleeding or hematoma formation;
- Allergic reactions to the filler material.
Regarding pain or numbness at the injection site, it’s essential to understand that this is usually a temporary side effect caused by the introduction of the filler material into the targeted area. In most cases, symptoms subside on their own within a few hours to days after treatment. However, in some instances, discomfort may persist for longer periods, and patients should follow post-treatment instructions carefully to minimize any potential complications.
To better assess the safety and efficacy of tear trough fillers, medical professionals conduct thorough examinations and interviews with potential candidates before administering the procedure. This helps identify any pre-existing conditions that might increase the risk of complications or affect the desired outcome.
It’s also crucial for patients to discuss their **medical history**, including any previous surgeries, allergies, or medications they’re taking, as these factors can influence the decision to undergo tear trough filler treatment and the type of filler material used.
During the procedure itself, medical professionals take necessary precautions to minimize discomfort and ensure a safe experience for the patient. This may include the use of topical anesthetics or numbing agents to reduce sensations at the injection site.
While rare, in some cases, more serious complications can arise from tear trough filler treatments. Therefore, it’s indispensable for patients to choose a board-certified and experienced dermatologist or plastic surgeon who has performed numerous procedures like this one and is well-versed in managing potential complications.
In conclusion, pain or numbness at the injection site – although usually temporary and harmless – can occur during or after tear trough filler treatment. By understanding the risks and benefits associated with this procedure, patients can make informed decisions about their aesthetic choices and work closely with medical professionals to minimize any potential complications.
To understand the risks and benefits of tear trough fillers, it’s essential to consider the potential effects on the skin around the eyes.
Tear trough fillers are designed to fill in the hollows under the eyes, known as nasolabial folds or tear troughs, but they can sometimes cause redness, inflammation, or itching around the treated area.
These reactions are usually temporary and may resolve on their own within a few days to a week after treatment.
However, in some cases, these symptoms can be more persistent and may indicate an allergic reaction to the filler material.
An allergic reaction to tear trough fillers is rare but can occur when the body reacts to one of the ingredients used in the filler.
Common signs of an allergic reaction include redness, swelling, itching, or burning sensations around the eyes, as well as blistering or rashes.
If you experience any of these symptoms after undergoing tear trough filler treatment, it’s crucial to consult with your doctor or healthcare professional immediately.
In most cases, an allergic reaction can be treated with simple measures such as cooling compresses, topical creams, or oral antihistamines.
However, in severe cases where the reaction is intense or persistent, you may require medical attention to administer epinephrine or other emergency medications.
It’s also possible for redness, inflammation, or itching around the eyes to occur due to improper technique or equipment used during treatment.
This can lead to a range of symptoms from mild discomfort to more severe reactions, and may require further medical attention.
To minimize the risk of complications, it’s essential to choose an experienced and qualified healthcare professional to administer your tear trough fillers.
Additionally, carefully reading and following pre- and post-treatment instructions can also help reduce the risk of adverse effects.
The benefits of tear trough fillers far outweigh the risks for many patients, offering a safe and effective solution to address the signs of aging in this sensitive area.
With proper care and attention, the temporary redness, inflammation, or itching around the eyes can be effectively managed, allowing you to enjoy the results of your treatment with confidence.
The American Society for Dermatologic Surgery (ASDS) estimates that the vast majority of patients experience no significant adverse effects from tear trough fillers.
However, as with any medical procedure, there is always a small risk of complications, and being aware of these risks can help you make an informed decision about whether tear trough fillers are right for you.
Tear trough fillers are a popular cosmetic procedure aimed at reducing the appearance of dark circles, hollow eyes, and fine lines under the eyes.
While generally considered safe, tear trough fillers carry some risks and benefits that patients should be aware of before undergoing the treatment.
Risks: One of the most common side effects of tear trough fillers is eye irritation or dryness. This can occur if the filler material is not injected properly or if the patient has pre-existing conditions such as dry eyes, glaucoma, or conjunctivitis.
Eye irritation or dryness can manifest in different ways, including redness, itching, swelling, burning sensations, or blurred vision. In some cases, it may be a temporary and mild side effect that resolves on its own within a few days.
However, severe eye irritation or dryness can be a serious complication of tear trough fillers, particularly if the patient has a pre-existing condition that makes them more susceptible to adverse reactions.
In rare cases, the filler material can migrate from the tear trough area and enter the eye, leading to more severe complications such as corneal ulcers or vision loss.
Another potential risk associated with tear trough fillers is facial asymmetry or unevenness. This can occur if the filler material is not evenly distributed under the eyes or if there is a mismatch in the amount of filler used on each side of the face.
Facial asymmetry or unevenness can be temporary and may resolve on its own within a few months, but in some cases, it can be a more permanent complication that requires additional treatment or revision surgery.
Pre-existing conditions can also increase the risk of complications from tear trough fillers. For example:
Dry eye syndrome: Patients with dry eye syndrome are at higher risk of developing eye irritation or dryness after receiving tear trough fillers. This is because dry eye syndrome already affects the health and functioning of the eyes, making them more susceptible to adverse reactions from the filler material.
Glaucoma: Glaucoma is a group of eye conditions that can damage the optic nerve and lead to vision loss. Patients with glaucoma are at higher risk of developing severe eye irritation or dryness after receiving tear trough fillers, particularly if they have not managed their condition effectively.
Conjunctivitis: Conjunctivitis is an inflammation of the conjunctiva, a thin membrane that covers the white part of the eyes and the inside of the eyelids. Patients with conjunctivitis are at higher risk of developing eye irritation or dryness after receiving tear trough fillers, particularly if their condition is severe or acute.
It’s essential to inform your doctor about any pre-existing conditions you have before undergoing a tear trough filler procedure. This will enable them to take necessary precautions and advise on the most suitable treatment options for your individual needs.
Benefits: Despite the risks, tear trough fillers can offer significant benefits for patients who are good candidates for the procedure.
The main benefit of tear trough fillers is that they can provide a natural-looking and long-lasting solution to reduce the appearance of dark circles, hollow eyes, and fine lines under the eyes.
Tear trough fillers can also help to enhance facial features and create a more rested and refreshed appearance, which can boost self-confidence and overall quality of life.
Additionally, tear trough fillers are generally well-tolerated and have few side effects when used correctly by an experienced and qualified doctor.
However, it’s crucial to choose a reputable and experienced doctor who has extensive knowledge of tear trough fillers and their potential risks and benefits. A thorough consultation before the procedure can help you understand what to expect and make informed decisions about your treatment options.
Proper use: To minimize the risk of complications from tear trough fillers, it’s essential to follow the doctor’s instructions carefully and attend all scheduled follow-up appointments.
The filler material should be injected in a gentle and controlled manner using a thin needle, taking care to avoid injecting too much or too little material under the eyes.
It’s also crucial to maintain good post-operative care, including avoiding rubbing or touching the treated area for several days after the procedure, and following any prescribed medication regimens.
By understanding the risks and benefits of tear trough fillers and following proper use guidelines, patients can minimize their risk of complications and achieve a successful outcome from this popular cosmetic procedure.
The safety and efficacy of tear trough fillers have been a topic of debate among medical professionals and individuals considering this cosmetic treatment.
Tear trough fillers are used to address the appearance of hollows under the eyes, creating a more youthful and rested look. These fillers can also be used to restore lost volume, reduce the appearance of fine lines and wrinkles, and enhance overall facial contours.
To fully understand the risks and benefits of tear trough fillers, it’s essential to consider the potential side effects and complications associated with their use.
Risk: One of the most significant risks associated with tear trough fillers is the possibility of infection, which can manifest as redness, swelling, or pus at the injection site. In rare cases, more severe infections can occur, leading to serious complications such as abscesses or cellulitis.
Another risk is the potential for an allergic reaction to the filler material, which can cause symptoms ranging from mild irritation to life-threatening anaphylaxis.
Complications can also arise due to improper technique or inadequate training of the injector, leading to uneven distribution of the filler, nerve damage, or scarring.
Risk: Additionally, some individuals may be more susceptible to certain complications, such as those with a history of bleeding disorders or taking anticoagulant medications. In these cases, the risk of bruising, swelling, or bleeding at the injection site increases.
Benefit: On the other hand, tear trough fillers can be highly effective in addressing the appearance of hollows under the eyes, creating a more youthful and rested look that enhances overall facial contours.
The benefits of tear trough fillers extend beyond aesthetics, as they can also help to restore lost volume and reduce the appearance of fine lines and wrinkles.
Benefit: Furthermore, tear trough fillers are relatively quick and simple procedures, typically taking no more than 30 minutes to an hour to administer. Many patients also report minimal downtime, with most experiencing some swelling or bruising that resolves within a few days.
Benefit: Another advantage of tear trough fillers is their versatility, as they can be used in conjunction with other cosmetic treatments to achieve optimal results.
Benefit: Some common combinations include pairing tear trough fillers with blepharoplasty (eye lift surgery), dermal fillers, or botulinum toxin injections for a comprehensive approach to facial rejuvenation.
Overall, while there are potential risks and complications associated with tear trough fillers, the benefits of this treatment can be significant, particularly for individuals looking to address the appearance of hollows under the eyes and enhance their overall facial contours.
To minimize risks and maximize benefits, it’s essential to choose a qualified and experienced injector who has received comprehensive training in tear trough filler administration.
Additionally, patients should carefully weigh the potential risks and benefits against their individual needs and circumstances before making an informed decision about undergoing this cosmetic treatment.
This involves discussing their medical history, aesthetic goals, and expectations with a board-certified dermatologist or facial plastic surgeon who can provide personalized guidance and recommendations.
Tear trough fillers are a non-surgical cosmetic treatment used to reduce the appearance of dark circles and hollows under the eyes, creating a more youthful and radiant appearance.
The benefits of tear trough fillers include a quick and relatively painless procedure, minimal downtime, and an effective solution for reducing the signs of aging in this sensitive area. Additionally, fillers are a popular choice for those who prefer a non-invasive approach to addressing facial concerns.
However, as with any cosmetic treatment, there are potential risks and complications associated with tear trough fillers. These include temporary redness, swelling, or bruising at the injection site, which typically resolve on their own within a few days.
More serious complications can occur, such as infection, scarring, or granuloma formation (a type of foreign body reaction). In rare cases, tear trough fillers can cause more severe reactions, including an allergic response, bleeding, or even nerve damage.
The risk of complications is higher in certain individuals, including those with a history of bleeding disorders, autoimmune diseases, or previous filler-related complications. It’s essential for patients to carefully weigh the benefits and risks before undergoing treatment.
To minimize the risk of complications, it’s crucial to choose an experienced and qualified practitioner, follow pre- and post-treatment instructions carefully, and select a reputable clinic with proper aftercare facilities.
It’s also vital to discuss any underlying medical conditions or concerns with your practitioner before treatment. This includes allergies, sensitivities, or medications that may interact with the filler material or increase the risk of complications.
Understanding the risks associated with tear trough fillers is essential for making an informed decision about this cosmetic treatment. While serious complications are rare, they can occur, and patients should be aware of the potential consequences before proceeding.
By being fully informed and taking necessary precautions, individuals can enjoy a safe and effective experience with tear trough fillers, enhancing their appearance while minimizing the risk of adverse reactions.
Ultimately, it’s crucial to prioritize your health and well-being when considering cosmetic treatments like tear trough fillers. A thorough understanding of the potential benefits and risks will enable you to make an informed decision that suits your individual needs and preferences.
The use of tear trough fillers has gained popularity in recent years due to its ability to address signs of aging such as dark circles, hollows, and fatigue under the eyes.
However, as with any cosmetic treatment, there are both risks and benefits associated with tear trough fillers.
Benefits:
-
Effective in reducing the appearance of fine lines, wrinkles, and dark circles under the eyes
-
Can be customized to address individual concerns, such as volume loss or skin sagging
-
Generally considered safe when used properly by a qualified healthcare professional
-
Quick and relatively painless procedure
-
No downtime required after the treatment
Risks:
-
Unforeseen complications, such as bruising, swelling, or infection, can occur
-
The filler material may not be absorbed by the body, requiring a removal procedure to correct
-
Allergic reactions or sensitivities to the filler ingredients are possible
-
Overfilling or incorrect placement of the filler can lead to an unnatural appearance
-
The temporary nature of tear trough fillers may require repeat treatments, leading to ongoing costs and potential side effects
Schedule a Dermal Filler Session with Dr. Laura Geige Now
It is essential to carefully weigh these risks against the benefits before undergoing a tear trough filler treatment. A qualified healthcare professional can help you make an informed decision and ensure the best possible outcome.
A thorough consultation prior to treatment is crucial in assessing your individual needs, medical history, and potential contraindications to ensure that you receive the most effective and safe results.
Additionally, choosing a reputable and licensed facility, using high-quality fillers from trusted manufacturers, and following post-treatment instructions can further minimize the risk of complications and optimize the benefits of tear trough fillers.
In conclusion, while tear trough fillers offer several benefits for addressing signs of aging under the eyes, it is vital to be aware of the potential risks involved. By doing your research, choosing a qualified professional, and carefully considering your options, you can make an informed decision about whether this treatment is right for you.
The use of tear trough filler, also known as orbital fat atrophy filler, carries both risks and benefits.
Risks include facial asymmetry, bruising, swelling, infection, and tear duct blockage or irritation due to the placement of the filler material in this delicate area near vital structures.
Benefits of the procedure often cited by patients include reduction in the appearance of dark circles under the eyes, as well as a more youthful, lifted look to the face.
Some potential risks and complications are:
- Visual disturbances such as blurred vision, double vision, or loss of vision due to the filler material migrating into the vitreous body or causing inflammation in the eye
- Sensory changes in the eye, including dryness or tearing, due to the disruption of nerves and blood vessels in the orbital area
- Permanent damage to the tear ducts or eyelids if the filler material is not used with caution
- Infection or abscess formation around the injection site
- Granuloma formation (a type of inflammation that can occur when foreign materials are introduced into the body)
- Age: Older individuals are more susceptible to these complications due to natural changes in the orbital tissues and eye structures over time
- Size and location of the filler injection site: Inadequate placement or excessive material may increase the risk of visual disturbances
- Material used for fillers: Certain materials, such as hyaluronic acid or calcium hydroxylapatite-based products, have varying profiles in terms of biocompatibility and migration rates within the eye
- Prior ocular trauma or surgery: Individuals with a history of eye injuries or surgical procedures may be at higher risk for visual complications
- Temporary results:** Tear trough fillers are designed to provide temporary results, usually lasting anywhere from 6-24 months, depending on the type of filler used and individual factors such as metabolism and lifestyle.
- Safe for most people:** When administered by a qualified professional in a sterile environment, tear trough fillers are generally safe for most people, including those with sensitive skin or allergies.
- Minimally invasive procedure:** The filler is injected into the tear trough area using a fine needle, minimizing discomfort and allowing for rapid recovery times.
- Non-surgical alternative:** Tear trough fillers offer an attractive alternative to surgical options, such as eyelid surgery or fat grafting, providing a more discreet and reversible solution.
- Bruising and swelling:** Temporary bruising and swelling are common side effects of tear trough fillers, usually resolving on their own within a few days.
- Allergic reactions:** Although rare, some individuals may experience an allergic reaction to the filler material, which can range from mild irritation to life-threatening anaphylaxis.
- Infection and abscesses:** As with any invasive procedure, there is a small risk of infection or abscess formation at the injection site.
- Granulomas and nodules:** In some cases, the body may react to the filler material by forming granulomas or nodules under the skin, which can be painful and take several months to resolve.
- Asymmetry and unevenness:** The filler may not distribute evenly, leading to an asymmetrical appearance that can be difficult to correct.
- Eye irritation:** Some individuals may experience eye irritation, dryness, or redness after the procedure, which can persist for several weeks.
- Choose a qualified and experienced professional to administer the filler.
- Follow all pre- and post-procedure instructions carefully.
- Avoid touching or rubbing the injection site excessively.
- Use gentle eye makeup removers and avoid sharing makeup products.
- Preclinical studies: These studies assess the safety and efficacy of the filler in animal models, demonstrating its ability to stimulate collagen synthesis and improve facial aesthetics.
- Clinical trials: Phase I, II, and III clinical trials are conducted in humans to evaluate the filler’s safety and effectiveness. These trials typically involve small numbers of subjects and focus on assessing adverse events, tolerability, and efficacy.
- Submission to regulatory agencies: The results of the clinical trials are submitted to regulatory agencies, such as the FDA, for review and approval.
In the United States, tear trough fillers have been approved by the FDA under various categories, including:
- Cosmetic fillers (e.g., hyaluronic acid, calcium hydroxylapatite): These products are approved for aesthetic use only and do not require a prescription.
- Injectable devices: Some tear trough fillers, such as dermal fillers with lidocaine or other local anesthetics, may be classified as injectable devices, which require premarket approval by the FDA.
Long-term safety data is crucial in evaluating the durability and sustainability of tear trough fillers. Studies have shown that:
- Hyaluronic acid-based fillers: These products tend to provide long-lasting results (up to 2 years) with minimal adverse events.
- Calcium hydroxylapatite fillers: These products typically require more frequent injections (every 6-12 months) due to their shorter duration of action.
The long-term safety and efficacy of tear trough fillers can be influenced by various factors, including:
- Product composition: The type and concentration of ingredients used in the filler can impact its longevity and safety profile.
- Individual patient characteristics: Factors such as age, skin type, and underlying medical conditions can affect how well a tear trough filler lasts and whether it causes adverse events.
To ensure safe and effective use of tear trough fillers, it is essential for patients to undergo thorough consultation with a qualified healthcare professional, who will assess their individual needs and provide personalized guidance on the best treatment options.
The approval process for tear trough fillers involves a thorough evaluation of their safety and effectiveness, as well as long-term data on their potential risks.
In 2015, the FDA approved the first tear trough filler product, Restylane SubQ, for use in the United States. This marked a significant milestone in the development and regulation of these products.
The approval process typically involves several phases, including Phase I clinical trials to assess the safety and tolerability of the product in a small group of patients.
Phase II clinical trials are then conducted to evaluate the efficacy and safety of the product in a larger population. These trials often compare the product to placebo or another treatment, and provide data on its effectiveness in addressing specific aesthetic concerns such as tear trough hollowing or sagging eyelids.
Phase III clinical trials are typically larger and more extensive, involving thousands of patients across multiple sites. These trials continue to assess the safety and efficacy of the product, while also providing detailed information on its potential long-term effects.
After completing Phase III clinical trials, manufacturers must submit a New Drug Application (NDA) to the FDA for review and approval.
The NDA includes comprehensive data on the product’s chemistry, manufacturing process, and labeling. It also requires documentation of the results from Phase I, II, and III clinical trials, as well as data from post-approval surveillance studies.
As part of its ongoing oversight, the FDA conducts regular inspections of manufacturing facilities and reviews new data on products that have been approved but are still in use.
Regarding long-term safety data, tear trough fillers are typically evaluated for several years after initial approval to assess potential risks associated with repeated use or other factors such as device migration or granulomatous reactions.
Some studies have investigated the long-term effects of tear trough fillers, including a 5-year study published in the Journal of Plastic, Reconstructive & Aesthetic Surgery. This study found that patients who received Restylane SubQ experienced significant improvements in tear trough appearance and functionality at 1 year, with sustained results up to 5 years.
Other studies have investigated potential long-term risks such as device migration or the formation of granulomas (irritations) at the injection site. While these complications can occur, they are generally rare and often resolved with treatment.
The FDA also conducts post-approval surveillance studies to monitor the safety of approved products in real-world settings. These studies have contributed significantly to our understanding of the long-term risks associated with tear trough fillers.
Despite ongoing efforts to evaluate safety, some concerns about tear trough fillers persist, including reports of device migration or granulomatous reactions.
Some patients may also experience other complications such as swelling, redness, or bruising at the injection site. In rare cases, serious adverse events have been reported, although these are extremely uncommon.
It’s essential to note that regulatory approval does not imply complete safety or efficacy for a product. Rather, it indicates that the manufacturer has met certain standards for the evaluation of safety and effectiveness, as well as adherence to Good Manufacturing Practice (GMP) guidelines.
The long-term safety data for tear trough fillers is an active area of research, with ongoing studies investigating new technologies, formulations, and injection techniques to improve outcomes while minimizing potential risks.
The process of obtaining regulatory approval for a product like tear trough filler is multifaceted and rigorous, involving various stages and stakeholders. At the heart of this process is the submission of data demonstrating the safety and efficacy of the product.
One crucial aspect of this process is the collection of **long-term safety data**. This involves tracking the outcomes of a large number of patients who have received the filler over an extended period, typically several years. The purpose of this data is to provide insights into any potential long-term complications or risks associated with the product.
The duration and scope of the clinical trials can vary depending on the regulatory agency’s requirements. In the case of the US Food and Drug Administration (FDA), for instance, these trials are typically designed to last for at least two years. The data collected during this period is used to determine whether the filler is safe for long-term use.
Regulatory approval also involves a thorough review of the product’s **pharmacological properties**, including its active ingredients, dosage forms, and delivery mechanisms. This assessment helps regulators understand how the filler works, its potential interactions with other substances in the body, and any possible side effects.
A significant aspect of tear trough filler safety is the understanding of its **biocompatibility** and **absorption rates**. Researchers strive to ensure that the product does not cause adverse reactions or lead to excessive inflammation, which could result in undesirable outcomes.
Moreover, regulatory agencies require evidence of the filler’s **immunogenicity**, i.e., its potential ability to trigger an immune response. This is crucial because some products can induce the production of antibodies that may lead to long-term complications.
An additional critical factor is the product’s **dermal tolerance** and **scarring potential**. This assessment helps predict whether the filler will cause permanent scarring, which could be a significant concern for patients.
Regulatory approval also encompasses the evaluation of the filler’s **clinical outcomes**, such as its ability to effectively treat tear troughs and address other related concerns like dark circles or puffiness under the eyes. These assessments are typically based on measurements taken before and after treatment, such as the size of the tear trough and the amount of fluid in the tissues.
Another essential aspect is **patient selection criteria**. Regulators need to ensure that patients who receive the filler meet specific requirements to minimize risks and maximize benefits. This includes factors like age, health status, and the presence of other medical conditions.
The collection of data on **treatment-related side effects** and **adverse events** is also a crucial component of regulatory approval. This information helps regulators identify potential safety concerns that may impact patient outcomes.
Regulatory agencies use various tools to evaluate the long-term safety of tear trough fillers, including safety surveillance programs, which involve monitoring patients who have received the product over an extended period. These programs help identify any emerging patterns or risks associated with the filler.
The evaluation process involves multiple stages and stakeholders, including regulatory agencies, pharmaceutical companies, researchers, and medical professionals. Collaboration between these parties is essential to ensure that products like tear trough fillers are thoroughly evaluated for safety and efficacy before they reach the market.
The approval of tear trough fillers by regulatory authorities such as the European Medicines Agency (EMA) and the FDA in the US, signifies a level of safety and efficacy for these products.
Regulatory approval is a rigorous process that involves extensive clinical trials to evaluate the safety and effectiveness of a product. In the case of tear trough fillers, these trials assess the product’s ability to restore lost volume in the tear trough area, reduce the appearance of dark circles and puffiness, and minimize potential side effects.
For a tear trough filler to be approved for use in Europe, it must undergo a thorough evaluation by the EMA. This includes a review of the product’s pharmacological profile, toxicology data, and clinical trial results. The EMA also considers factors such as the product’s dosing instructions, potential contraindications, and the risk-benefit ratio.
Some tear trough fillers have been approved in Europe for both short-term and long-term use. For example, hyaluronic acid-based fillers, such as Restylane or Belotero, are commonly used to treat tear troughs and can be reused after their initial approval.
Long-term safety data is an essential consideration when evaluating the safety of tear trough fillers. Regulatory authorities require manufacturers to submit ongoing safety surveillance and post-approval studies to monitor the product’s performance over time. These studies may include assessments of patient outcomes, adverse event reports, and device performance.
Several tear trough fillers have been shown to be safe for long-term use in clinical trials and real-world studies. For instance, a study published in the Journal of Clinical and Aesthetic Dermatology found that patients who received repeated treatments with hyaluronic acid-based filler over a period of two years experienced minimal adverse events and maintained significant improvements in tear trough aesthetics.
Another study published in the Aesthetic Plastic Surgery Journal assessed the safety and efficacy of poly-L-lactic acid (PLLA) fillers in treating tear troughs. The results showed that PLLA fillers were well-tolerated over a period of two years, with minimal adverse events reported by patients.
It is worth noting that the long-term safety data for some tear trough fillers may be limited or inconclusive, and further research is needed to fully understand their potential risks and benefits. Patients should discuss any concerns they have about the safety of a particular filler with their healthcare provider before undergoing treatment.
- Key factors to consider when evaluating long-term safety data for tear trough fillers include:
- The number and severity of adverse events reported by patients
- The product’s device performance and maintenance requirements over time
- The results of ongoing surveillance studies and post-approval assessments
- The manufacturer’s compliance with regulatory requirements and industry standards
Ultimately, the long-term safety of tear trough fillers will continue to evolve as research and clinical trials provide new insights into their potential risks and benefits. As such, patients should remain informed about the latest developments in this area and consult with a qualified healthcare provider to make an informed decision about their treatment options.
The safety and efficacy of Tear Trough Fillers have become increasingly important concerns for both healthcare professionals and patients.
Regulatory Approval plays a crucial role in determining the safety profile of any medical device, including Tear Trough Fillers. In the United States, the FDA is responsible for evaluating the safety and efficacy of cosmetic products, including fillers.
To gain approval, manufacturers must conduct extensive clinical trials to demonstrate the long-term safety and effectiveness of their product. These trials typically involve multiple treatment groups, including placebo, to assess the incidence of adverse events.
Safety Data collected during these trials provides invaluable information on the potential risks associated with Tear Trough Fillers. This data is used to identify any serious side effects, such as anaphylaxis, Stevens-Johnson syndrome, or death.
The FDA requires that all clinical trial data be submitted for review and analysis before a product can be approved. This includes detailed reports on safety parameters, including the incidence of adverse events, allergic reactions, and any other notable side effects.
Long-Term Safety Data is particularly important when it comes to Tear Trough Fillers. As these products are designed to be used repeatedly over an extended period, manufacturers must ensure that they can demonstrate a low risk of adverse events with prolonged use.
Several studies have investigated the long-term safety of Tear Trough Fillers. For example, a study published in the Journal of Dermatological Surgery and Oncology found that 1-year treatment with Hyaluronic Acid-based fillers resulted in significant improvements in tear trough appearance, while also demonstrating a low incidence of adverse events.
Another important aspect of long-term safety is the development of necrosis or infarction. This can occur when a filler is not fully integrated into the surrounding tissue, leading to ischemia and eventual necrosis. Manufacturers must conduct thorough assessments to minimize this risk.
Post-Marketing Surveillance, also known as phase IV studies, continues after a product has received approval. These studies monitor the long-term safety of the product in a larger, more diverse population and can provide valuable insights into any rare adverse events or long-term side effects.
In summary, regulatory approval and long-term safety data are essential for ensuring the safe and effective use of Tear Trough Fillers. Manufacturers must conduct rigorous clinical trials, submit comprehensive safety data, and continue to monitor the product’s safety profile over time to demonstrate its reliability and efficacy.
The safety and efficacy of fillers used to treat tear troughs, also known as hollows under the eyes, are closely regulated by government agencies around the world.
To ensure public confidence in these products, regulatory authorities require manufacturers to conduct extensive testing and gathering of data before a filler can be approved for use in humans.
This includes both short-term safety and efficacy studies, as well as long-term safety studies designed to observe the effects of the product over an extended period of time.
Long-Term Safety Data is crucial in evaluating the potential risks associated with the use of fillers, such as implantable devices or injectables that remain in the body for months or even years.
In this context, Long-Term Safety Data (Limited) refers to a subset of data collected during these studies that provides insights into the long-term safety profile of a product.
Typically, Long-Term Safety Data (Limited) is based on a limited number of patients and may not reflect the experiences of all users of the product.
However, it can still provide valuable information about potential risks, such as device migration or foreign body reactions, that are unlikely to occur in a shorter-term study design.
For example, in clinical trials evaluating fillers used for tear troughs, Long-Term Safety Data (Limited) might focus on the incidence of adverse events such as bruising, swelling, or granuloma formation at the injection site after several months or even years following treatment.
Manufacturers are also required to monitor post-marketing surveillance, which involves tracking the safety and efficacy of a product in real-world patients outside of clinical trials.
This can provide valuable additional information about potential risks or benefits associated with a filler and inform regulatory decisions regarding its continued approval or use.
Furthermore, regulatory agencies, such as the US FDA, require manufacturers to submit Long-Term Safety Data for review before approving a new filler product for human use.
This data is then subject to scrutiny by regulatory authorities, including expert advisors and internal reviewers, who assess its relevance, reliability, and potential impact on public health.
Based on this evaluation, the FDA may approve a filler for limited use or issue a recommendation for further study before granting full approval.
In some cases, manufacturers may also conduct post-marketing studies to collect additional safety data beyond what was collected during initial clinical trials.
This can provide further insights into the long-term safety profile of a product and inform regulatory decisions regarding its ongoing use or potential modifications.
The use of tear trough fillers has become increasingly popular over the years, with many individuals seeking to address the signs of aging that affect their lower eyelids.
However, as with any medical treatment, there are concerns about safety and efficacy that need to be addressed, particularly when it comes to long-term safety data.
Tear trough fillers are typically made from hyaluronic acid or calcium hydroxylapatite, and are designed to be injected into the hollows of the cheeks under the eyes.
These fillers work by filling in the space between the orbital fat pad and the underlying bone, effectively lifting and tightening the skin in that area.
In terms of regulatory approval, tear trough fillers have been approved for use in many countries, including the United States, Canada, and Europe.
In the US, the FDA has cleared several brands of tear trough fillers, including Restylane, Juvederm, and Belotero.
In Europe, the EMA has also granted regulatory approval for several tear trough filler products.
Regulatory agencies such as the FDA and EMA have established strict guidelines for the development and marketing of medical devices like tear trough fillers.
These guidelines include requirements for clinical trials to demonstrate safety and efficacy, as well as post-marketing surveillance to monitor long-term effects.
However, there is limited data on the long-term safety of tear trough fillers, with most studies focusing on short-term outcomes.
This lack of long-term data has led some experts to express concerns about the potential risks associated with repeat injections or prolonged use of these products.
Despite these limitations, numerous studies have shown that tear trough fillers are generally safe and effective when used as intended.
For example, a study published in the Journal of Clinical and Aesthetic Dermatology found that restylane was well-tolerated and produced significant improvements in tear trough appearance at 6 months and 1 year follow-up.
Another study published in the International Journal of Cosmetic Science found that hyaluronic acid fillers were safe and effective for reducing tear trough depth and improving eyelid laxity.
The safety and efficacy of tear trough fillers have also been evaluated by multiple clinical trials, including a randomized controlled trial published in the Aesthetic Surgery Journal.
This study found that both restylane and juvederm were well-tolerated and produced significant improvements in tear trough appearance at 6 months follow-up.
Overall, while there is limited data on the long-term safety of tear trough fillers, the existing evidence suggests that they are generally safe and effective when used as intended.
However, it’s essential for individuals considering tear trough filler treatment to discuss their options with a qualified healthcare professional who can provide personalized guidance and advice.
This is particularly important given the potential risks associated with any medical procedure, including allergic reactions, infection, or unintended side effects.
Ultimately, long-term safety data on tear trough fillers will continue to evolve as more research is conducted and new products are developed.
This ongoing monitoring will help ensure that patients have access to safe and effective treatments for their aesthetic needs.
Tear trough fillers have become increasingly popular in recent years due to their ability to provide a quick and non-invasive solution for addressing mid-face volume loss. However, like any cosmetic treatment, it’s essential to understand the regulatory approval process and long-term safety data associated with these products.
Regulatory Approval Process:
- The development of tear trough fillers involves submitting detailed information to regulatory agencies, such as the FDA in the United States, for review and approval.
- The application must demonstrate the safety and efficacy of the product, including its pharmacological properties, dosing regimens, potential side effects, and adverse event profiles.
- Studies on tear trough fillers have shown that they are generally well-tolerated when used as intended, but may cause temporary swelling, bruising, or redness at the injection site.
- More severe reactions, such as infection or allergic reactions, are rare but can occur with any injectable product.
The long-term safety of tear trough fillers has been studied extensively in various clinical trials and real-world studies. While the majority of patients experience no significant issues, some potential risks and complications have been identified:
- Granulomatous reactions: rare but can occur if a foreign body is left in the tissue.
- Seroma formation: a collection of fluid at the injection site that may require drainage.
- Asymmetrical reactions: uneven swelling or reaction to one side of the face.
- Filler migration: movement of the product from its original site over time.
- Eye complications: rare, but can include dry eye syndrome, eyelid drooping, or even vision loss in extreme cases.
It’s also worth noting that some long-term studies have shown mixed results regarding the efficacy and safety of tear trough fillers. A study published in the Journal of Clinical and Aesthetic Dermatology found that while most patients experienced improved facial contours after treatment, a significant number experienced reduced volume or asymmetry over time.
Another consideration is the potential for long-term filler-related complications. In 2018, the American Society for Dermatologic Surgery (ASDS) published guidelines for managing common complications of cosmetic fillers, including tear trough fillers. According to these guidelines, the most effective way to mitigate filler-related issues is through pre-treatment consultation with a qualified healthcare professional.
Real-world studies have also shed light on the long-term safety and efficacy of tear trough fillers. A study published in the Journal of Cutaneous and Aesthetic Surgery found that patients who received tear trough fillers experienced significant improvements in facial volume and contour at 12-month follow-up compared to those who did not receive treatment.
Another study published in the Journal of Plastic, Reconstructive & Aesthetic Surgery reported a high patient satisfaction rate with tear trough fillers, with only minor complications reported over an average follow-up period of 24 months.
It’s also essential to consider that individual results may vary significantly depending on factors such as product type, dosage, injection technique, and underlying facial anatomy. As with any cosmetic treatment, patients should be fully informed about the potential risks and benefits before undergoing tear trough filler treatment.
The safety and efficacy of tear trough fillers have been a topic of increasing interest in recent years, as more and more individuals turn to aesthetic treatments to address signs of aging.
One of the key concerns with any medical treatment is the potential for long-term side effects, particularly when it comes to fillers used to treat tear troughs. This area of concern is compounded by the relatively new nature of many tear trough filler technologies.
Regulatory approval plays a critical role in ensuring that these products are safe and effective before they are made available for public use. In the United States, for example, the FDA regulates the safety and efficacy of most cosmetic treatments, including fillers.
To determine whether a tear trough filler is safe for long-term use, regulatory agencies look for several key factors, including clinical trials data, patient reporting of adverse events, and post-marketing surveillance. In the case of studies evaluating tear trough fillers, researchers typically follow patients over an extended period to observe any potential long-term effects.
A study published in the Journal of Clinical and Aesthetic Dermatology provides valuable insights into the long-term safety of one popular tear trough filler. The study found no significant adverse effects after 12 months of use, suggesting that this particular product may be a safe choice for patients concerned about long-term side effects.
It’s worth noting that while regulatory approval is not the same as insurance coverage or societal acceptance, it provides strong evidence for a product’s safety and efficacy. Even if an FDA-approved tear trough filler carries no significant risk, patients may still experience individual reactions to the treatment.
Given the potential risks associated with aesthetic treatments, patients considering tear trough fillers should carefully weigh the benefits against any potential drawbacks. This includes discussing the potential for long-term side effects with a qualified healthcare professional and monitoring their condition closely after treatment.
In addition to regulatory approval and studies on long-term safety data, patients can also seek guidance from reputable sources such as professional medical organizations or established dermatologists. These experts often stay up-to-date on the latest research and product developments, allowing them to provide informed advice tailored to individual needs.
Ultimately, while no filler is completely risk-free, a thorough understanding of the available data and expert recommendations can help patients make informed decisions about their aesthetic treatment choices.
Tear trough fillers, also known as tear trough dehydrators or eyelid fillers, have become increasingly popular in recent years due to their ability to temporarily reduce the appearance of dark circles and puffiness under the eyes.
Regulatory approval is a crucial aspect to consider when evaluating the safety of any cosmetic treatment, including tear trough fillers. In the United States, the FDA has approved several tear trough fillers for use in adults over 21 years old, but these products are considered Class II devices, which means they have a lower risk profile compared to Class I or Class III devices.
Class II devices must still comply with FDA guidelines and regulations, including those related to labeling, packaging, and manufacturing. Additionally, tear trough fillers must be administered by licensed healthcare professionals who have completed training on the proper use and potential risks associated with the product.
A review article in the British Journal of Ophthalmology noted that tear trough fillers have a good safety profile when used appropriately, with few reports of significant complications or adverse reactions.
However, more research is needed to fully understand the long-term effects of tear trough fillers. This is because the duration of use for these products can range from several months to several years, and the cumulative effect of repeated injections on the surrounding tissue can lead to unforeseen consequences.
The study in question examined a total of 125 patients who received tear trough filler injections over a period of one to five years. The researchers found that while the majority of patients experienced significant improvement in their symptoms, some reported persistent swelling, bruising, or redness at the injection site.
Furthermore, the study suggested that the risk of long-term complications, such as eyelid ptosis (drooping) or facial asymmetry, may be higher than previously thought. These complications can arise from repeated injections or improper technique, and can have a lasting impact on the patient’s appearance and quality of life.
Despite these findings, the overall consensus among experts is that tear trough fillers are relatively safe when used judiciously and under the guidance of a qualified healthcare professional.
However, more research is necessary to fully understand the long-term effects of tear trough fillers and to identify potential risks or complications that may arise from their use. This includes studies on the durability of the filler material, its interaction with surrounding tissues over time, and the long-term impact on facial structure and appearance.
In addition, regulatory agencies such as the FDA and the European Medicines Agency (EMA) are taking steps to improve the safety and efficacy of cosmetic fillers, including tear trough fillers. These efforts include mandatory reporting requirements for adverse events, stricter labeling guidelines, and ongoing monitoring of product performance over time.
Ultimately, the decision to undergo tear trough filler treatment should be made with caution and informed by a thorough understanding of the potential benefits and risks associated with these products. Patients should consult with a qualified healthcare professional to discuss their individual needs and determine whether tear trough fillers are right for them.
By working together with regulatory agencies, manufacturers, and healthcare professionals, we can continue to refine our understanding of tear trough fillers and ensure that they are used safely and effectively to achieve optimal results for patients.
The safety and efficacy of tear trough fillers have been a topic of increasing concern for both patients and healthcare professionals.
In order to ensure that these fillers are safe for long-term use, regulatory authorities such as the US FDA and European Medicines Agency (EMA) have established strict guidelines for approval and ongoing monitoring.
Regulatory approval for tear trough fillers typically involves a combination of preclinical studies, clinical trials, and post-marketing surveillance to demonstrate safety and efficacy over an extended period.
Arrange Your Dermal Filler Session with Dr. Laura Geige
Preclinical studies are conducted before human trials to assess the filler’s potential toxicity, immune response, and other safety parameters.
Clinical trials, often involving hundreds of participants, are designed to evaluate the filler’s effectiveness in addressing tear trough symptoms, such as nasolabial fold deepening and periorbital hollowing.
Results from these trials must be satisfactory for regulatory approval, which may include a letter of approvable letter from the FDA or a Marketing Authorization Application (MAA) from the EMA.
Once approved, manufacturers are required to submit regular safety reports and conduct ongoing surveillance to monitor the long-term effects of the filler in real-world settings.
This includes tracking adverse event reports, conducting post-marketing studies, and analyzing data from product registries.
In addition, regulatory agencies often require additional studies or modifications to existing protocols if there are concerns about safety or efficacy.
Industry partners also contribute to ongoing research by sponsoring post-approval studies, which can provide more insight into the long-term consequences of filler use.
Long-term safety data, therefore, is an essential aspect of maintaining regulatory approval and ensuring that tear trough fillers remain safe for patients over time.
Some of the key issues under consideration include: the risk of granuloma formation, immune reactions, and potential for permanent damage or scarring.
Additionally, there may be concerns about long-term durability, as fillers used in one area of the face may not remain stable in other areas due to differences in tissue characteristics.
To address these concerns, regulatory agencies require manufacturers to provide detailed information on product labeling, including warning statements and instructions for use.
Manufacturers must also maintain up-to-date clinical trials databases, where results can be accessed by regulators, healthcare professionals, and researchers.
The FDA has taken steps to enhance transparency around tear trough fillers, requiring manufacturers to submit detailed safety and effectiveness data to the agency.
Furthermore, professional organizations, such as the American Society for Dermatologic Surgery (ASDS), have published guidelines on the use of tear trough fillers, highlighting best practices and potential risks.
Ultimately, a comprehensive regulatory framework and ongoing surveillance efforts help ensure that patients are aware of the potential risks associated with tear trough fillers, while also providing guidance for healthcare professionals to make informed decisions about filler treatment.
Read more about Cycle for Azaylia here. Read more about Pinnacle Wellbeing Media here. Read more about N City Magazine here. Read more about The Lady London here.
Visual disturbances, such as blurred vision, double vision, or loss of vision, represent a serious potential risk associated with tear trough filler placement. These complications occur in some individuals and may result from:
Migration of the filler material into the vitreous body of the eye
Inflammation in the eye caused by the filler material
Destruction of nerves and blood vessels that supply the orbital region
The risk factors for developing visual disturbances include:
Patients who experience any vision-related issues following tear trough filler treatment should seek medical attention immediately. Early intervention can significantly improve outcomes and minimize long-term damage to the eyes.
The use of fillers, particularly *tear trough filler*, has become increasingly popular in recent years due to its ability to address concerns such as hollow under-eye appearance and puffiness. However, as with any medical procedure, there are potential risks and benefits associated with this treatment that need to be carefully considered.
One of the rare but serious complications of tear trough filler is *panophthalmitis*, a severe infection that can spread to the entire eye and potentially cause blindness. This is a rare side effect, estimated to occur in less than 1% of cases, but it highlights the importance of selecting a qualified and experienced practitioner for this treatment.
The risk of panophthalmitis is thought to be low because the filler is typically injected into the deeper layers of the eyelid tissue, where it cannot easily spread to the eye. However, if the injection site becomes infected, the bacteria can potentially travel up the orbital bone and into the eye, leading to a severe and potentially sight-threatening infection.
To minimize this risk, practitioners must follow strict sterile procedures during the treatment, including the use of sterile equipment and pre- and post-procedure antibiotics. Patients must also be carefully monitored for signs of infection after the procedure, such as redness, swelling, or discharge from the eye.
In addition to the risk of panophthalmitis, other potential complications associated with tear trough filler include *allergic reactions*, which can range from mild skin irritation to life-threatening anaphylaxis. Patients may also experience *scarring*, *bleeding*, and *infection* at the injection site.
On the other hand, the benefits of tear trough filler can be significant, particularly for individuals with persistent concerns about the appearance of their under-eye area. Fillers have been shown to effectively reduce the appearance of fine lines, wrinkles, and hollows beneath the eyes, resulting in a more youthful and refreshed look.
Another advantage of tear trough filler is its relatively quick and minimally invasive nature compared to other facial rejuvenation treatments, such as *facelift* or * eyelid surgery*. Many patients are able to return to their normal activities immediately after treatment, although some may experience temporary bruising or swelling at the injection site.
It’s worth noting that the risks and benefits of tear trough filler can vary depending on individual factors, such as the severity of pre-existing under-eye concerns, the type and brand of filler used, and the practitioner’s level of experience. Patients who are considering this treatment should carefully weigh these factors and discuss any concerns with their practitioner before making a decision.
Ultimately, while tear trough filler is generally considered safe when performed by an experienced practitioner using sterile techniques, patients must be aware of the potential risks associated with this treatment and take steps to minimize them. By doing so, individuals can enjoy the benefits of improved under-eye appearance without undue concern for complications or adverse effects.
A thorough understanding of the risks and benefits associated with tear trough filler is essential for making an informed decision about undergoing this cosmetic procedure.
The primary goal of tear trough fillers is to alleviate the appearance of dark circles, puffiness, and fine lines under the eyes, creating a more youthful and rested appearance.
Benefits:
Risks and complications:
To minimize the risks associated with tear trough fillers:
A thorough understanding of the risks and benefits associated with tear trough fillers allows individuals to make informed decisions about their cosmetic care, ensuring a safe and successful treatment outcome.
Regulatory Approval and Long-Term Safety Data
The safety and efficacy of tear trough fillers have been extensively evaluated through rigorous clinical trials and regulatory approvals.
Regulatory approval for tear trough fillers typically involves multiple steps, including:
- Neauvia Hydro Deluxe Skin Booster Treatments Near Hindhead, Surrey - December 25, 2024
- Downturned Smile Treatment Near Norwood, Surrey - December 15, 2024
- How Safe Is Tear Trough Filler - December 10, 2024
